OUI Medical announces completion cadaver study and clarence form Institutional Review Board for clinical study.
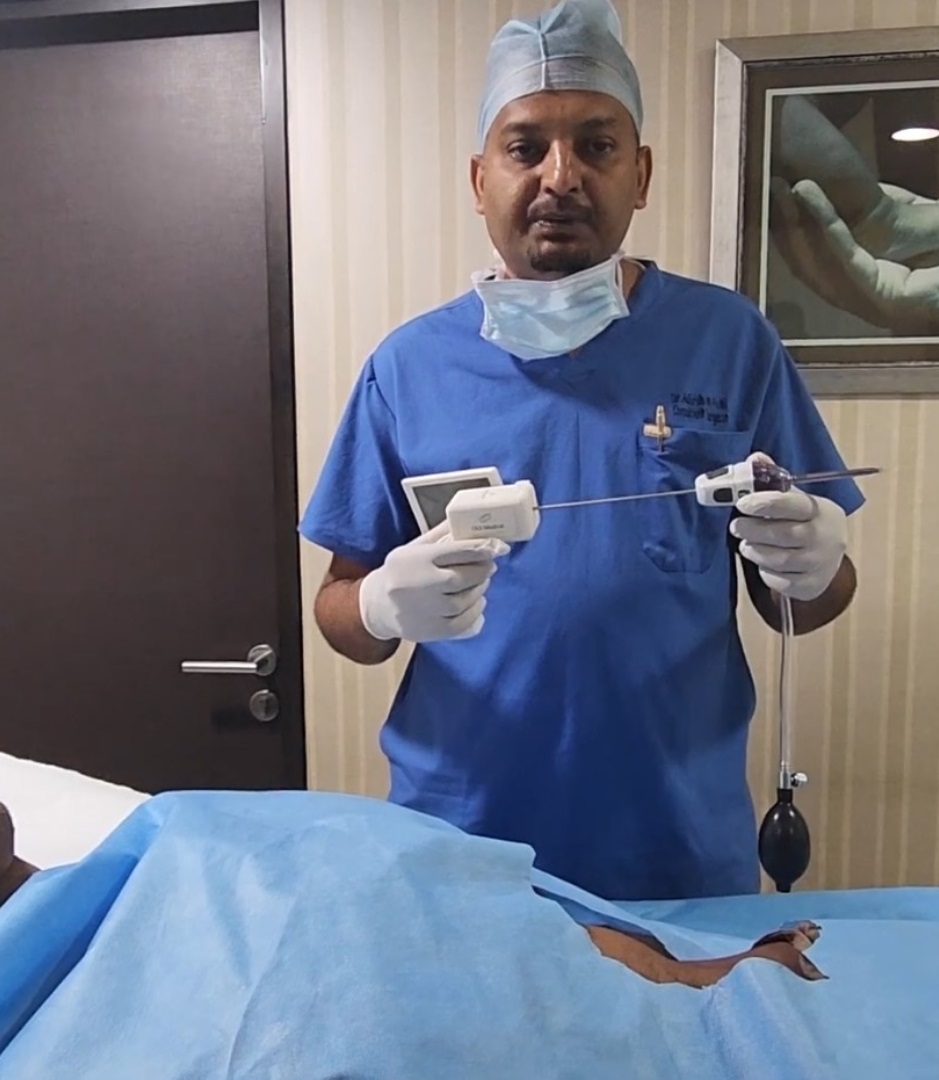
[Bangalore ] – 10-2-2023 – A new medical device has been developed that promises to revolutionize the way Peritoneoscopy is performed. The Peritoneoscope, a portable handheld scope with an integrated camera, screen, processor, battery, and biopsy forceps, has completed a cadaver study and has been cleared by the Institutional Review Board for a clinical study.
Peritoneoscope is a minimally invasive procedure that is used to examine the inside of the abdomen. It is typically performed under general anaesthesia in a hospital or surgery centre setting. However, with the Peritoneoscope, Peritoneoscope can now be performed at the point of care under local anaesthesia or local anaesthesia with sedation.

The Peritoneoscope is a disposable device, which eliminates the need for reprocessing and reduces the risk associated with cross-contamination. Additionally, the low acquisition cost of the device makes it an affordable option for medical facilities of all sizes.
“We are thrilled to announce the completion of our cadaver study and clearance from the Institutional Review Board for a clinical study,” said the inventor of the Peritoneoscope. “Our goal with this device is to make Peritoneoscopy more accessible, less invasive, and more affordable for patients and medical facilities alike. We are excited to move forward with our clinical study and continue to improve the future of medical care.”
The clinical study for the Peritoneoscope is expected to begin April. The results of the study will be used to further refine the device and bring it one step closer to commercial availability.
For more information about the Peritoneoscope, please contact
Dr Adarsh M Patil ap@oui-medical.com or visit www.oui-medical.com .